Collagen: On Wound Healing and some Pathologies
COLLAGEN
a fibrous protein, like elastin
elastin is rich in glycine, proline, and alanine
collagen is the most abundant protein in mammals, approx 30% of total body prot
both found in skin, CT, BV's, scler and cornea of eye
the major fibrous component of CT (connective tissue)
collagen may be dispersed as a gel (vitreous humor) or bundled paralell (tendon)
in the cornea collagen is stacked to transmit light w/o scattering
bone collagen fibers are angled to each other
TYPES
20+ types
types of collagen distinguished by different amino acid chains used: alpha 1, 2, 13, etc.
types I, II, III are fibrillar, rope-like, linear
type I collagen in skin, bone, tendon has greatest tensile strength, 80% more than type III
type II more cartilaginous
type III more distensible, found in BVs, early scar tissue
types IV and VII form 3D mesh, are major part of basement membranes
types IX and XII bind to sfc of collagen fibrils, linking them to each other and more
STRUCTURE
prevalent aminos: glycine (1/3), proline
approximately half of the collagen sequence is not glycine, proline or hydroxyproline
proline or hydroxyproline constitute about 1/6 of the total sequence
proline facilitates helical formation because it causes kinks in the chain
usual sequence is GLY - X - Y
where X is usu proline and Y is often hydroxyproline or hydroxylysine
thus (GLY - PRO - HYP - ) x 333 = one chain
chain: structural components approx 1,000 aminos long
a triple helix (L handed, unlike DNA) of cross-linked alpha chains
helix held together by hydrogen bonding among amino acids
some amino acid side chains on surface to allow binding with neighboring monomers-->long fiber
hydroxyproline and hydroyxline not seen in most prots
made by hydroxylation of proline and lysine after their incorporation into the polypeptide
hydroxyl group of hydroxylysine may be enzymatically glycosylated
often gluc & galact attached before triple helix formation
(does this happen more with high serum glucose levels?)

MANUFACTURE OF COLLAGEN
protein chains assembled in nucleus of fibroblasts, osteoblasts, chondroblasts
delivered to rough endoplasmic reticulum for cleavage, hydroxylation
enzymes: prolyl oxidase and lysyl hydroxylase (requires oxygen and vitamin C)
after hydroxylation is glycosylation
disulfide bonds on C-terminal extensions line up chains for twisting
you then have procollagen
procollagen exported from cell via Golgi apparatus-->secretory vesicles-->release into ECM
after export procollagen is cleaved by N- and C-procollagen peptidases
now have triple-helical tropocollagen molecules which spontaneously associate to form fibrils
a regular staggered but parallel pattern
fibrillar array is substrate for LYSYL OXIDASE (contains copper)
this enzyme oxidatively deaminates some lysyl and hydroxylysyl residues
aldehydes resulting are reactive and condense with lysyl or hydroxylysyl residues in neighbors
forming covalent cross-links-->mature collagen
DEGRADATION OF COLLAGEN
very stable molecule, half life is years
breakdown depends on collagenases
type I collagen has specific cleavage site
WOUND REPAIR BY FIBROSIS
occurs when injury is severe or persistent
tissue in 3rd deg burn cannot be restored to normal dt loss of skin, BM, CT structure
WOUND HEALING BY FIRST INTENTION
day 1: fibrin clot, neuts come in to liquefy injuried tissue, increased mitotic activity of basal cells in squamous epithelium around wound margines
day 2: squamous cells from neighboring basal cell layer migrate under the fibrin clot and seal off the wound after 48 hours, macrophages come in
day 3: granulation tissue begins to form. Initial deposition of type III collage begins but does not bridge incision site. macrophages replace neutrophils.
days 4-6: granulation tissue formation peaks, collagen bridges incision site
week 2: collagen compresses BV's in fibrous tissue, reducing blood flow, tensile str = 10%
month 1: collagenase (Zn cofactor) remodeling of wound occurs with replacement of type III collagen by type I. Tensile strength increases. Scar tissue is devoid of adnexal structures (hair, glands) and inflammatory cells
SECOND INTENTION
different healing
more intense inflammatory rxn
increased granulation tissue formation
wound contraction caused by increased numbers of myofibroblasts
PATHOLOGIES OF COLLAGEN FORMATION
SCURVY
vitamin C (ascorbic acid) deficiency
lysyl-oxidase is the enzyme that crosslinks collagen strands
vitamin C-->hydroxylation of proline and lysine in initial collagen synthesis by fibroblasts
sites of hydroxylation are anchor sites for cross links between triple helix of alpha chains
collagen is weak
gums bleed, poor wound healing
EHLERS-DANLOS SYNDROME
a group of mendelian disorders
defects in type I and III fibrillar collagen synthesis/structure
enzymes that may be deficient incl: lysyl hydroxylase, procollagen peptidase
also can result from mutations of aa sequences in types I, III, or V (III most imp)
mutant collagen is not secreted and accumulates in fibroblast
arteries are weakened
Sx: hypermobile joints, ecchymoses, poor wound healing, hyperelasticity of skin
aortic dissection is mc cause of death

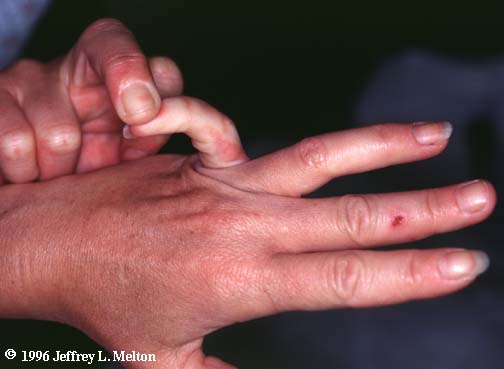

...now I understand why some of those circus performers can do the stuff they can do.
OSTEOGENESIS IMPERFECTA
aka brittle bone syndrome
bones esaily bend and fracture
delayed wound healing
rotated and twisted spine, humpback common
type I OI is osteogenesis imperfecta tarda
presents in early infancy wiht fractures dt minor trauma
may have prenatal bowing or fractures
type II OI is osteogenesis imperfecta congenita
more severe, pts die of pulmonary hypoplasia in uteria or during neonatal period
KELOID
bulky overformed scars
excess type III collagen formation
common in African Americans
may occur dt 3rd degree burns
microscopic: irreg, thick collagen bundles extending beyond border of injury
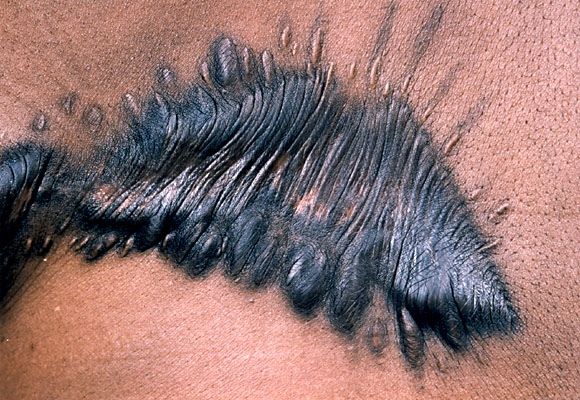


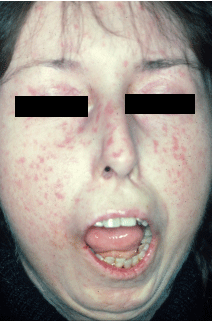
SYSTEMIC SCLEROSIS (see ch3)
mc cause of death is lung dz
excessive collagen production dt auto-immune rxn
mostly in skin but also GI (esophagus), lungs, kidneys
women of childbearing age
small vessel EC damage-->BV fibrosis and ischemic injury
T-cells release cytokines-->excessive collagen synthesis
Sx: Raynaud's is 1st sign, skin atrophy and tissue swelling begins in fingers and extends proximally, parchment-like appearance, extensive dystrophic calcifications in subcu, tightened facial features, dysphagia for solids and liquids (bottom 2/3 of esph smooth m replaced by collagen), small bowel looses villi-->malabsorption, wide-mouthed diverticuli-->bacterial overgrowth, interstitial fibrosis of lungs, renal vasculitis of arterioles and glomeruli (hyperplastic arteriolosclerosis), infarctions, malignant HTN
LABS: serum ANA+ 70-90%, anti-topoisomerase Ab+ in 15-40%
CREST syndrome: calcific, centomere ab, raynauds, esoph dysmot, sclerodactyly, telangiectasias
crest shows anti-centomere ab's in 30%


IDIOPATHIC PULMONARY FIBROSIS
~15% of cases of chronic interstitial lung dz
more common in males
usu over 40 years, up to 70
repeated cycles of alveolitis triggered by unknown agent
release of cytokines-->interstitial fibrosis-->proximal dilation of small airways
honeycomb appearing lung tissue
NOT COLLAGEN PATHOLOGY
MARFAN SYNDROME
autosomal dominant weakness of elastic tissue
defect in fibrillin = component of elastic tissue
fibrillin is a leukocyte adhesion molecule (integrins and selectins are made of it)
ocular: dislocated lens
twisted humped spine much like OI
aortic dissection

SOURCES
Rapid Review Pathology, Goljan
Wikipedia
Biochemistry; Lippincott's Illustrated Reviews