(no subject)
Is anybody up and good at science? I am having issues understanding the difference between miscible or immiscible mixtures. I understand that miscible means they don't separate, but IDK how to tell if a mixture IS miscible. Like I have:
a.) H20 and CBr4
b.) SiO2 and BF3
c.) Br2 and CBr4
etc., and it's asking me if they are miscible or immiscible. And idk what to base it on. Do I have to look at their polarity? Or what. Her stupid slideshow does not fucking tell me. It just says "like dissolves like" and this doesn't help me at all. This is literally all she says on the subject:
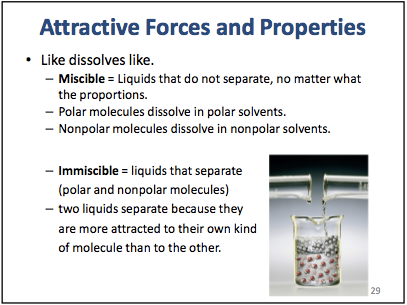
What does this meannnnnnn. Also. I hate Lewis structures and this valence shell order nonsense. It's so much work and math for such a tiny answer, lol.
EDIT: Nevermind, I think I got it. However, if you have an easier way of explaining this, I would love to hear it because I have a test on Tuesday lmao.
a.) H20 and CBr4
b.) SiO2 and BF3
c.) Br2 and CBr4
etc., and it's asking me if they are miscible or immiscible. And idk what to base it on. Do I have to look at their polarity? Or what. Her stupid slideshow does not fucking tell me. It just says "like dissolves like" and this doesn't help me at all. This is literally all she says on the subject:

What does this meannnnnnn. Also. I hate Lewis structures and this valence shell order nonsense. It's so much work and math for such a tiny answer, lol.
EDIT: Nevermind, I think I got it. However, if you have an easier way of explaining this, I would love to hear it because I have a test on Tuesday lmao.